URIDINE SIDE EFFECTS AND SAFETY

It is important to understand that the safety profile of uridine is relatively poorly known and researched, as there are few well-designed clinical trials. Therefore, the following list of side effects is not exhaustive:
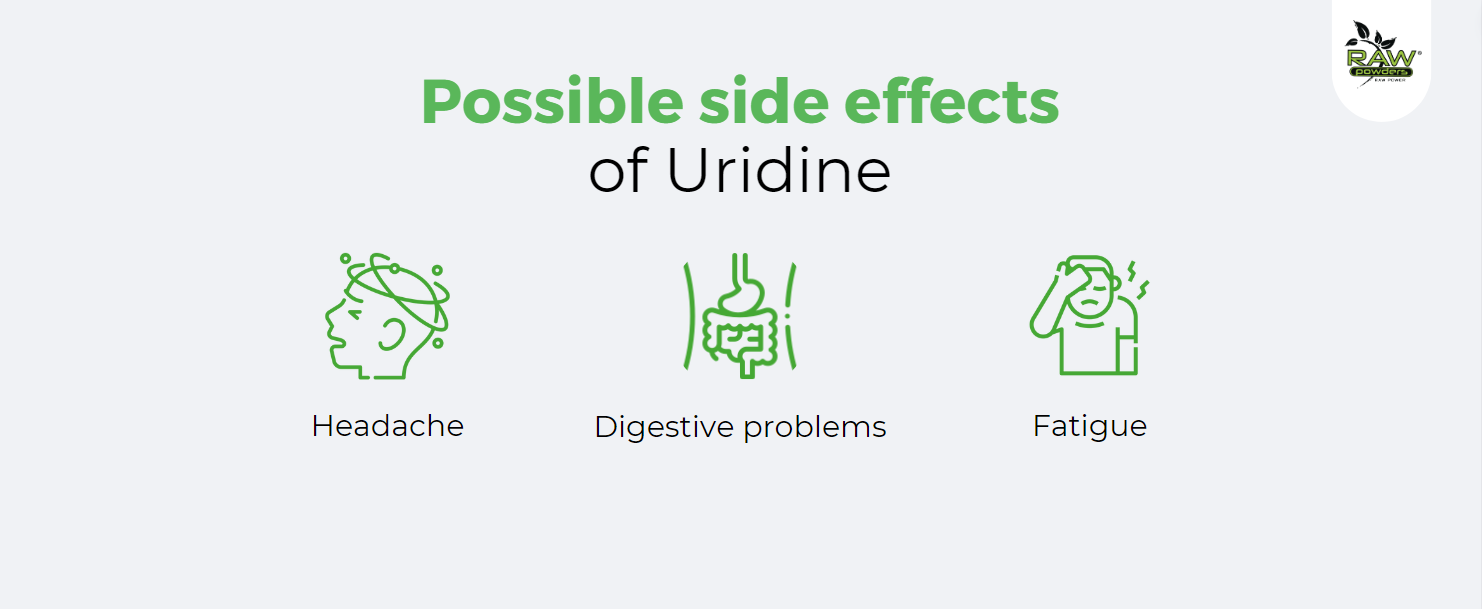
- Digestive Issues: Some people might experience digestive issues such as nausea, diarrhea, or stomach upset.
- Fatigue: There have been reports of fatigue or drowsiness in some individuals taking uridine.
- Headaches: Uridine can potentially cause headaches in some users.
For other possible side effects, you should consult your doctor, taking into account your medical condition and possible drug or supplement interactions.
Implications of clinical trials
In one clinical study, uridine did not cause any side effects in 9 healthy people taking uridine for one week. One person lost consciousness, but according to the researchers, this may not have been due to uridine supplementation [1].
During the study, with high doses (300 mg) of uridine monophosphate, there were no serious adverse events or allergic reactions. No clinically significant deviations of laboratory parameters were recorded [2].

There are no warnings or precautions associated with the use of uridine triacetate, although data regarding the safety of uridine triacetate during pregnancy in humans are insufficient. When administered to pregnant rats at half the maximum recommended human dose, no teratogenicity or effects on embryo fetal development occurred [3].
There is no data regarding the presence of uridine triacetate in human breast milk or its effect on the breast-fed infant or breast milk production.
The safety and effectiveness of uridine triacetate in pediatric patients have been established, but are based on a small number of patients in open-label studies.
The safety and effectiveness of uridine triacetate in patients 65 years and older have not been established because studies did not include sufficient numbers of patients older than 65 years to determine if they respond differently from younger patients.
The most common adverse reactions associated with uridine triacetate in clinical trials were vomiting (10%), nausea (5%), and diarrhea (3%). Overall, uridine triacetate was well tolerated.
Uridine triacetate is a weak inhibitor of P-glycoprotein (P-gp), but due to the potential for high drug concentrations in the GI tract, it may interfere with narrow therapeutic index P-gp substrates (eg, digoxin). In vivo data in humans are not available [3].
In depressed adolescents, the side effects of uridine were mild and included vivid dreams, mild abdominal cramps, nausea, diarrhea and indigestion [4].
One review reported that high doses of uridine can also cause fever in humans [5].

Health risks
Preliminary studies have linked high uridine intake to a number of health conditions. Most of the studies have been carried out in animals and cells, which means that uridine may not have the same effects in humans. However, it can be assumed that the possible health risks of uridine can be:
1. Cancer
Uridine supplementation may increase the risk of cancer through activation of the P2Y2 receptor. P2Y2 activation is beneficial in autoimmune diseases but may increase cancer growth. Several cell-based studies have shown that P2Y2 activation increases cancer progression in both human and animal cells [6, 7, 8, 9, 10].
One possible way in which uridine can induce cancer is by incorporating the uracil nucleotide into DNA instead of thymine [11, 12]. Folate and B12 deficiency can cause the same problem. If this is the case, sufficient folate and vitamin B-12 should help counteract the carcinogenic potential of uridine [13].
Another study reveals that pancreatic cancer cells can use uridine as a fuel source when glucose is scarce. This ability is linked to the expression of the enzyme uridine phosphorylase 1 (UPP1). The research shows that uridine-derived ribose is essential for maintaining the redox balance and survival of cancer cells in glucose-restricted environments, suggesting that targeting uridine utilization could be a novel therapeutic approach for treating pancreatic cancer [14].
The findings, published in Nature, show that cancer cells can adapt when they don’t have access to glucose. Researchers have previously identified other nutrients that serve as fuel sources for pancreatic cancer; this study adds uridine to the catalog [15].
2. Cardiac scarring
In rats, uridine triphosphate (UTP) induced scarring of cardiac tissue (by activating P2Y2 receptors). The scarring thickens the heart valves, making the heart muscle less efficient at pumping blood. Scarring affecting a large area of the heart can lead to heart failure [16].
3. Insulin resistance
In a human study, patients with type 2 diabetes had higher concentrations of uridine compared to healthy subjects. Elevated uridine levels correlated with insulin resistance as it was associated with high levels of insulin, C-peptide and HOMA-IR [17].
In studies with rats, high doses of uridine injected into the bloodstream caused insulin resistance. Uridine has also been shown to increase insulin secretion in human cells [17, 18].
4. Decrease in bone density
Uridine triphosphate (UTP) can reduce bone density, but the evidence is conflicting. In one cell-based study, UTP strongly inhibited bone mineralisation and bone formation (via P2Y2 receptor activation) [19, 20].
In another cell-based study, uridine triphosphate prevented bone formation and increased the number of fat cells in the bone marrow, which in turn reduces bone strength [21].
However, another cell study found that uridine triphosphate promoted bone mineralisation [22].
The data on whether uridine promotes bone loss or bone formation are conflicting. If you have osteoporosis or are at risk of developing osteoporosis, it would be better to avoid taking uridine for the time being.
Uridine is a naturally occurring organic compound belonging to the group of nucleosides essential for the synthesis of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). It plays a crucial role in the genetic information and cellular functions, including energy metabolism.
When considering uridine supplements, it is important to consider the potential benefits and appropriate dosage. Although this article discusses the potential side effects, uridine also has positive properties. Read more about this in the blog post Uridine benefits.
It is important to stress that dosage is crucial and should be determined according to individual needs and health conditions. It is always advisable to consult a doctor before taking uridine or any other supplement. Only a qualified professional can help determine the appropriate dosage, taking into account individual health and lifestyle factors. For recommendations on the use and dosage of uridine supplements, see here.
Medical Disclaimer
The information provided in our articles is solely for educational purposes and should not be considered medical advice or instruction. No action or inaction should be taken based solely on the contents of this information. Readers should consult their health care professional on any matter related to their health and well-being. The information and opinions provided here are believed to be accurate and sound, based on the best judgment available to the authors, but readers who fail to consult with appropriate health authorities assume the risk of any injuries. The publisher is not responsible for errors or omissions.
Please be aware that different countries may have specific regulations and that this disclaimer does not replace the need for consultation with a healthcare provider before beginning or changing a treatment or supplement regimen. The information contained in this article is not intended to diagnose, treat, cure, or prevent any disease. Individual results may vary.
References
- Nivedita Agarwal, Young-Hoon Sung, J. Eric Jensen, Grace daCunha, David Harper, David Olson, Perry F. Renshaw. Short-term administration of uridine increases brain membrane phospholipids precursors in healthy adults: a 31-phosphorus magnetic resonance spectroscopy study at 4T. Bipolar Disord. Author manuscript; available in PMC 2011 Dec 1. Published in final edited form as: Bipolar Disord. 2010 Dec; 12(8): 825–833. doi: 10.1111/j.1399-5618.2010.00884.x. PMCID: PMC3020593. NIHMSID: NIHMS254875. PMID: 21176029. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3020593/
- E. Z. Yakupov, J. V. Troshina, R. R. Gainutdinova, A. O. Kashapova. [The results of a study on the safety of the dietary supplement neurouridine in patients with nonspecific low back pain (MULTINEURO-2)]. Zh Nevrol Psikhiatr Im S S Korsakova. 2021;121(3):52-56. doi: 10.17116/jnevro202112103152. PMID: 33834718. https://pubmed.ncbi.nlm.nih.gov/33834718/
- Dennis J. Cada, Uzoma Mbogu, Ross J. Bindler, Danial E. Baker. Uridine Triacetate. Hosp Pharm. 2016 Jun;51(6):484-8. doi: 10.1310/hpj5106-484. PMID: 27354750 PMCID: PMC4911989. https://pubmed.ncbi.nlm.nih.gov/27354750/
- Douglas G. Kondo, Young-Hoon Sung, Tracy L. Hellem, Kristen K. Delmastro, Eun-Kee Jeong, Namkug Kim, Xianfeng Shi, Perry F. Renshaw. Open-Label Uridine for Treatment of Depressed Adolescents with Bipolar Disorder. J Child Adolesc Psychopharmacol. 2011 Apr; 21(2): 171–175. doi: 10.1089/cap.2010.0054. PMCID: PMC3080753. PMID: 21486171. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3080753/
- Arpád Dobolyi, Gábor Juhász, Zsolt Kovács, Julianna Kardos. Uridine function in the central nervous system. Review Curr Top Med Chem. 2011;11(8):1058-67. doi: 10.2174/156802611795347618. PMID: 21401495. https://pubmed.ncbi.nlm.nih.gov/21401495/
- Zhe Cao, Jun Ma, Xinchun Chen, Boping Zhou, Chuan Cai, Dan Huang, Xuewen Zhang, Deliang Cao. Uridine homeostatic disorder leads to DNA damage and tumorigenesis. Cancer Letters. Volume 372, Issue 2, 28 March 2016, Pages 219-225. https://doi.org/10.1016/j.canlet.2016.01.007. https://www.sciencedirect.com/science/article/abs/pii/S0304383516000197
- Ji Hun Choi, Young Geon Ji, Dong Hyeon Lee. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas. 2013 May;42(4):680-6. doi: 10.1097/MPA.0b013e318271bb4b. PMID: 23462325. https://pubmed.ncbi.nlm.nih.gov/23462325/
- Hana Jin, So Young Eun, Jong Sil Lee, Sang Won Park, Jae Heun Lee, Ki Churl Chang, Hye Jung Kim. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014 Aug 26;16(5):R77. doi: 10.1186/bcr3694. PMID: 25156554 PMCID: PMC4406012. https://pubmed.ncbi.nlm.nih.gov/25156554/
- Wei-Hua Li, Ying Qiu, Hong-Quan Zhang, Xin-Xia Tian, Wei-Gang Fang. P2Y2 Receptor and EGFR Cooperate to Promote Prostate Cancer Cell Invasion via ERK1/2 Pathway. PLoS One. 2015 Jul 16;10(7):e0133165. doi: 10.1371/journal.pone.0133165. eCollection 2015. PMID: 26182292 PMCID: PMC4504672. https://pubmed.ncbi.nlm.nih.gov/26182292/
- Rui Xie, Jingyu Xu, Guorong Wen, Hai Jin, Xuemei Liu, Yuan Yang, Bei Ji, Yixia Jiang, Penghong Song, Hui Dong, Biguang Tuo. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem. 2014 Jul 4;289(27):19137-49. doi: 10.1074/jbc.M113.540047. Epub 2014 May 20. PMID: 24847054 PMCID: PMC4081950. https://pubmed.ncbi.nlm.nih.gov/24847054/
- N Li, X T Zhou. Human chromosome hot points. IV. Uridine-induced hot-point breaks at 3p14 and 16q23-24 and increased expression of fragile site Xq27 in folate-free medium. Hum Genet. 1985;71(4):363-5. doi: 10.1007/BF00388465. PMID: 4077052. https://pubmed.ncbi.nlm.nih.gov/4077052/
- SN Wickramasinghe, S. Fida. Bone marrow cells from vitamin B12- and folate-deficient patients misincorporate uracil into DNA. Blood (1994) 83 (6): 1656–1661. https://doi.org/10.1182/blood.V83.6.1656.1656. https://ashpublications.org/blood/article/83/6/1656/170921/Bone-marrow-cells-from-vitamin-B12-and-folate
- Benjamin C. Blount, Matthew M. Mack, Carol M. Wehr, +5, and Bruce N. Ames. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. PNAS April 1, 1997. 94 (7) 3290-3295. https://doi.org/10.1073/pnas.94.7.3290. https://www.pnas.org/doi/10.1073/pnas.94.7.3290
- Zeribe C. Nwosu, Matthew H. Ward, Peter Sajjakulnukit, Pawan Poudel, Chanthirika Ragulan, Steven Kasperek, Megan Radyk, Damien Sutton, Rosa E. Menjivar, Anthony Andren, Juan J. Apiz-Saab, Zachary Tolstyka, Kristee Brown, Ho-Joon Lee, Lindsey N. Dzierozynski, Xi He, Hari Ps, Julia Ugras, Gift Nyamundanda, Li Zhang, Christopher J. Halbrook, Eileen S. Carpenter, Jiaqi Shi, Leah P. Shriver, Gary J. Patti, Alexander Muir, Marina Pasca di Magliano, Anguraj Sadanandam, Costas A. Lyssiotis. Uridine-derived ribose fuels glucose-restricted pancreatic cancer. Nature. 2023 Jun;618(7963):151-158. doi: 10.1038/s41586-023-06073-w. Epub 2023 May 17. https://pubmed.ncbi.nlm.nih.gov/37198494/
- Anna Megdell. Study finds cancer cells use a new fuel in absence of sugar. Nature (May 17, 2023. https://www.michiganmedicine.org/health-lab/study-finds-cancer-cells-use-new-fuel-absence-sugar
- Oscar O. Braun, David Lu, Nakon Aroonsakool, Paul A. Insel. Uridine triphosphate (UTP) induces profibrotic responses in cardiac fibroblasts by activation of P2Y2 receptors. J Mol Cell Cardiol. 2010 Sep;49(3):362-9. doi: 10.1016/j.yjmcc.2010.05.001. Epub 2010 May 13. PMID: 20471392 PMCID: PMC2917486. https://pubmed.ncbi.nlm.nih.gov/20471392/
- Tetsuya Yamamoto, Taku Inokuchi, Tuneyoshi Ka, Asako Yamamoto, Sumio Takahashi, Zenta Tsutsumi, Daisuke Tamada, Chiharu Okuda, Yuji Moriwaki. Relationship between plasma uridine and insulin resistance in patients with non-insulin-dependent diabetes mellitus. Nucleosides Nucleotides Nucleic Acids. 2010 Jun;29(4-6):504-8. doi: 10.1080/15257771003740986. PMID: 20544544. https://pubmed.ncbi.nlm.nih.gov/20544544/
- Fariborz Parandeh, Sandra Meidute Abaraviciene, Stefan Amisten, David Erlinge, Albert Salehi. Uridine diphosphate (UDP) stimulates insulin secretion by activation of P2Y6 receptors. Biochem Biophys Res Commun. 2008 Jun 6;370(3):499-503. doi: 10.1016/j.bbrc.2008.03.119. Epub 2008 Apr 1. PMID: 18387359. https://pubmed.ncbi.nlm.nih.gov/18387359/
- Astrid Hoebertz, Siva Mahendran, Geoffrey Burnstock, Timothy R Arnett. ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: a novel role for the P2Y2 receptor in bone remodeling. J Cell Biochem. 2002;86(3):413-9. doi: 10.1002/jcb.10236. PMID: 12210747. https://pubmed.ncbi.nlm.nih.gov/12210747/
- Isabel R. Orriss, Gillian E. Knight, Sam Ranasinghe, Geoffrey Burnstock, Timothy R. Arnett. Osteoblast responses to nucleotides increase during differentiation. Comparative Study Bone. 2006 Aug;39(2):300-9. doi: 10.1016/j.bone.2006.02.063. Epub 2006 Apr 17. PMID: 16616882. https://pubmed.ncbi.nlm.nih.gov/16616882/
- Wenkai Li, Sheng Wei, Chaoxu Liu, Mingyu Song, Hua Wu, Yong Yang. Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: The role of P2Y2 receptor and ERK1/2 signaling. Int J Mol Med. 2016 Jan; 37(1): 63–73. Published online 2015 Nov 3. doi: 10.3892/ijmm.2015.2400. PMCID: PMC4687443. PMID: 26531757. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4687443/
- Victoria B. Ayala-Peña, Luis A. Scolaro, Graciela E. Santillán. ATP and UTP stimulate bone morphogenetic protein-2,-4 and -5 gene expression and mineralization by rat primary osteoblasts involving PI3K/AKT pathway. Exp Cell Res. 2013 Aug 1;319(13):2028-2036. doi: 10.1016/j.yexcr.2013.05.006. Epub 2013 May 23. PMID: 23707969. https://pubmed.ncbi.nlm.nih.gov/23707969/





_front%20(1)-250x250.png)



_front%20(1)-250x250.png)

-(NN)_front%20(1)-min-250x250.png)

_front%20(1)-250x250.png)


_front%20(1)-min-250x250.png)
_front%20(1)%20(1)-250x250.png)
_front%20(1)%20(1)-250x250.png)
_front%20(1)-min-250x250.png)
_front%20(1)-min-250x250.png)
_front%20(1)%20(1)-250x250.png)
_front%20(1)%20(1)-250x250.png)
_front%20(1)-min-250x250.png)

